Introduction
The nitrogen cycle is the circulation of nitrogen between living organisms and the environment.
Whilst there is an abundance of nitrogen within the atmosphere, this is mostly unavailable to plants.
Nitrogen Fixation
Atmospheric nitrogen can be utilised, i.e. fixed, by micro-organisms into ammonium which is then incorporated (assimilated) by them as organic nitrogen. These micro-organisms either live freely within the soil and are called non-symbiotic bacteria, or they live in association with some plants, for example many leguminous plants which have a nitrogen fixing bacteria, Rhizobium, within their root nodules. Clover is a common turf weed that is a leguminous plant.
Nitrification
Fertilisers applied to turf are broken down, through the activities of nitrifying bacteria, into readily available nitrates which are assimilated by plants.
Ammonium is oxidised (broken down through the addition of oxygen) by the action of Nitrosomonas species; the resulting nitrite is further oxidised by the action of Nitrobacter species into nitrate which can be taken up by a plant from the soil solution.
Nitrification has a number of requirements for optimum working. The following is a general guide:
- pH to be about 7.0; The process can be reduced to less than 10% of its optimum in very acidic soils;
- temperature to be between 30 and 35° C; negligible activity occurs below 5°C;
- moisture content to be about 60% of field capacity;
- oxygen supply to be adequate.
One of the reasons why different fertilisers take different amounts of time to break down and finally be utilised by the grass plant is due not only to the formulation in which the nitrogen is held within the fertiliser, but also soil conditions which can significantly influence the nitrification process.
Denitrification
If conditions are unsuitable for the oxidation of nitrite to nitrate, or if such conditions arise after nitrate has been produced, then denitrifying bacteria act on the nitrite or nitrate resulting in the loss of nitrogen (N2) and nitrous oxide (N2O)gas back to the atmosphere.
Denitrification occurs under certain conditions:
- waterlogged or poorly drained soils;
- most active around pH 7.0;
- a large amount of readily decomposable organic matter;
- high soil temperatures.
Ammonification
Ths is the breakdown of complex organic molecules within dead and decaying organic matter to ammonium, which is the start of the nitrification process.
Mineralisation
This is sometimes used to refer to the combined processes of nitrification and ammonification, whilst sometimes is refers just to the ammonification process.
Nitrogen losses
Nitrate, which is negatively charged, is not held onto soil or organic particles (which are also essentially negatively charged) within the soil and can be easily leached out of the soil if irrigation or rainfall exceeds the field capacity of the soil.
Ammonium, on the other hand, is positively charged and is not as easily leached as nitrates from soil based rootzones.
High sand content sports pitch rootzones will readily leach ammonium as the sand, with effectively no charge, is unable to hold onto the ammonium ions.
Losses also occur through the removal of clippings from a mown sward.
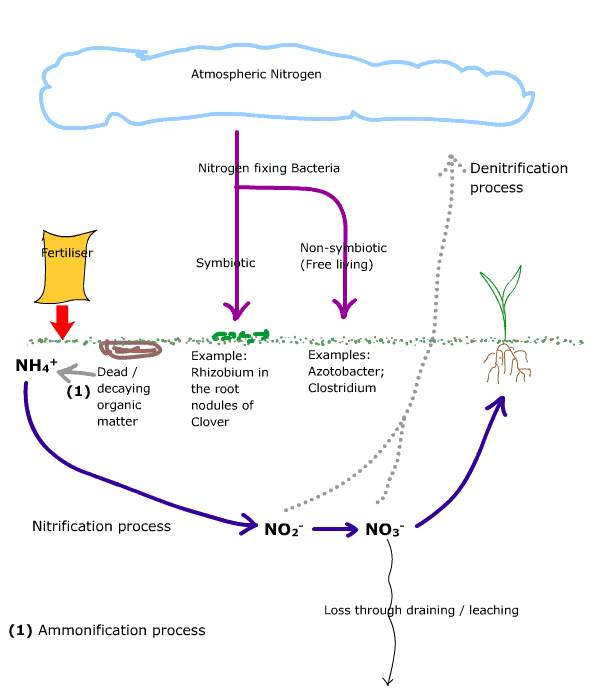
Key to diagram:
- NH4+= ammonium;
- NO2-= nitrite;
- NO3- = nitrate
References and further reading
- Hanson, A.A. & Juska, F.V. (Eds), (1969), 'Turfgrass Science', Agronomy No. 14, American Society of Agronomy; 108-116
- White, R.E. (1979), 'Introduction to the Principles and Practice of Soil Science', Blackwell Scientific Publications, 104-109, 131-136
- Stevenson, F.J. (Ed), (1982), 'Nitrogen in Agricultural Soils', Agronomy No.22, ASA, CSSA, SSSA, 940
- Adams, W.A. & Gibbs, R.J. (1994), 'Natural Turf for Sport and Amenity', CAB International, 34-37
- Rowell, D.L. (1994), 'Soil Science: Methods and Applications', 218-220
- Bailey, J. (Ed), (1999), 'The Penguin Dictionary of Plant Sciences', 504
- Taiz, L. & Zeiger, E. (2002), 'Plant Physiology', 259-272
