
Written by Sabine Braitmaier, Dr Deborah Cox and Dr Kate Entwistle
Photo credit Roger Clarke, AGS
In August 2020, the fungal disease Gray Leaf Spot (GLS) was, for the first time, confirmed as the cause of damage to a stadium pitch in the UK (Photo.1) and a notice was published in the Grounds Management magazine to make turf mangers aware of this potentially devastating disease. This article adds detail to that notice, and provides information on the fungus that causes the disease, describes the developing symptoms, outlines current control options and discusses potential options for disease management.

Photo.1 General symptoms of GLS on L. perenne (photo: R Clark, AGS)
Let’s start with the fungus
All living organisms have a so-called Latin name; a scientific name that places the organism within a specific genus and species. By doing this, we group together organisms that share similar characteristics so that we know all organisms within a certain genus, for example, will look or act in a similar way. Within a genus, some individuals may show differences in form or function and for that reason, we allocate a species name to show that there are slight differences present. We can further separate the species into sub-species, getting ever more focussed on the detailed differences of individual organisms. This orderly classification helps us to understand new or previously undescribed organisms in the early days of their discovery. In the living world, fungi have been unique in that they have always been ascribed two Latin names – one for each of their sexual and asexual stages. For the fungus that causes GLS disease, the genus name of the sexual stage is Magnaporthe and that of the asexual stage is Pyricularia. However, it was recently decided that fungal classification should be brought in line with all other organisms and there should be only one Latin name, regardless of the sexual/asexual stage of the fungus and that meant deciding which of the two names was the most appropriate. Although research has confirmed sexual reproduction of the fungus is possible in the laboratory, the sexual stage of the fungus has not (to our knowledge) been confirmed as occurring in nature (Wei, 2015). Quite possibly, for that reason, it has been decided that the genus name for the causal fungus should be Pyricularia and the species that specifically infects amenity turfgrasses should be P. oryzae.
Although the currently accepted name for the fungus that causes GLS in managed amenity turf is Pyricularia oryzae (Tharreau et al, 2019), there are many published articles and scientific papers that have used the names Pyricularia grisea, Magnaporthe grisea or Magnaporthe oryzae. It is likely that these ‘alternative’ names will continue to be used by some authors going forward in articles about GLS in turf, but what is important to know is that they are all talking about the same disease problem.
What turfgrasses can be affected by P. oryzae?
GLS is a fungal disease that can occur in warm- and cool-season turfgrasses and was initially identified in 1991 following the outbreak of a serious blight disease on a Perennial Ryegrass (Lolium perenne) fairway in the USA. Most notably, St. Augustinegrass (Stenotaphrum secundatum) and Kikuyugrass (Pennisetum clandestinum) are the most affected warm season grasses and Perennial Ryegrass (L. perenne) and Tall Fescue (Festuca arundinacea) the most affected, commonly used, cool season turfgrasses. Due to its extensive use, not only in sports fields (as natural pitches and in various hybrid turf systems) but increasingly in golf courses and other amenity areas, L. perenne is arguably the most important turfgrass to be affected by this disease. GLS can develop aggressively in young plants and 4-5 week old seedlings are extremely susceptible to infection.
What are the symptoms of infection?
The disease symptoms will vary slightly depending on the grass type affected and the age of infected plants. The most severe outbreaks tend to develop in newly established swards and they are a major concern where areas are regularly re-seeded with L. perenne. It is important to mention that initial symptoms of GLS disease develop on turf areas that receive the most amount of sunlight and that shaded or part-shaded turf area are noticeably less affected.
The initial symptoms are small, dark brown spots or lesions (1mm to 3mm diameter) on the leaf & stem tissues that may appear ‘watersoaked’. These lesions have a purple-coloured margin and may also have a yellowish zone of leaf tissue surrounding them (Uddin et al 2003). Individual leaves may become twisted although this symptom is not always easy to see in closer mown turf (Photo.2).

Photo.2 Leaf symptoms of GLS infection on L. perenne (photo: S Braitma ier)
When the disease is active, the number of lesions on a leaf will increase and individual lesions will enlarge rapidly. In warm-season turf, infected leaves will die and become brown, giving an overall dark cast to the sward but in cool-season turf, the leaf infection develops in to patches of dead turf that rapidly expand and coalesce (Butler & Kerns, 2019). In the early stages of the disease, symptoms may be confused with those of heat or drought stress and in the later stages the disease can easily be misidentified as Pythium blight. In affected L. perenne turf, the stem base tissue often becomes dark brown in colour (Photo.3) and can lead to a costly misdiagnosis of Basal Rot Anthracnose disease.

Photo.3 L. perenne plant with dark stem base (photo: Andy Cole, iTurf Management)
Active GLS outbreaks can result in the partial or complete destruction of a stadium pitch within 3 to 5 days. One unmistakable symptom of this active disease is the development of a countless number of spores on the affected plant tissues. The spores are held away from the plant surface on specialised mycelial structures called sporophores and each sporophore will support several spores. The individual spores are pear-shaped and contain three separate cells. Their characteristic shape makes microscopic diagnosis of active disease quick and decisive. The mass of spores produced from each lesion causes the development of a gray, ‘velvet-like’ covering to the affected tissues (Photo.4) and this symptom gives the disease its common name. Overnight incubation of infected plants under increased humidity will encourage this mass of spore production and can be a way of confirming a suspected disease outbreak. Symptoms of GLS can potentially be mistaken for those caused by Pythium fungi but with GLS, no obvious aerial fungal mycelium will develop on incubated plants.

Photo.4 Spore development on the leaf surface (photo: K Entwistle)
How does the fungus infect the plant?
GLS is foliar fungal disease. Spores of the causal fungus that come in to contact with the leaf surface will eventually adhere to the leaf and germinate (Figure 1). The spores produce a germination tube that quickly develops into a specialised infection structure known as an appressorium (Photo.5).
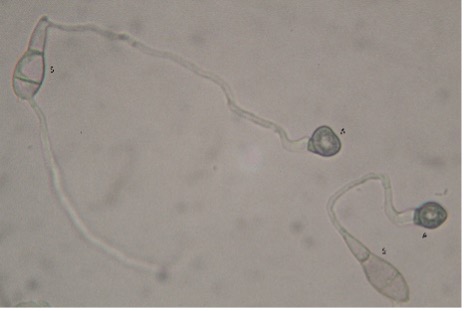
Photo. 5 Germinating spores (S) showing germ tubes with appressoria (A) (photo: K Entwistle)
As the appressorium develops, it darkens due to the accumulation of melanin that strengthens the structure and eventually, through a build-up of pressure within, the fungus forces its way inside the plant cell to which it is attached.
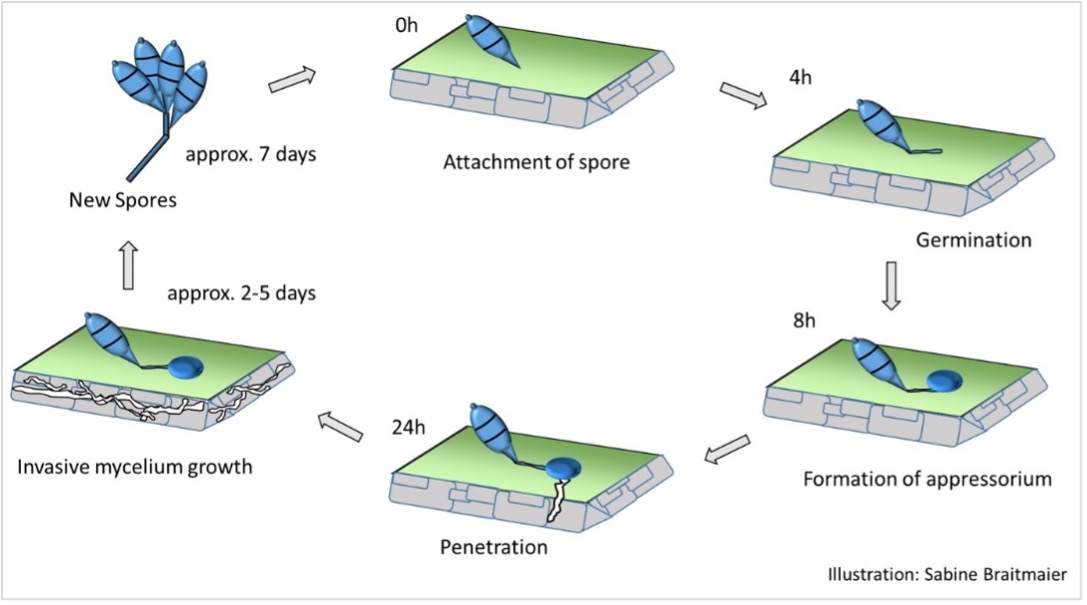
Figure 1
Once the fungus is inside the plant cell, the fungal mycelium develops through the plant tissues, utilising the plant cell content as a source of nutrition. This fungal development causes the observed ‘water soaking’ of the plant. As infection continues, the lesions mature, darken and expand, eventually causing the leaf to die. The fungus completes its life cycle by growing out of the dying plant tissues and producing spores on the plant surface. Spore development on infected plant tissues can take between 2 – 5 days (Uddin et al. 2003) but in general, during active disease development in the field, samples of infected plants that are incubated overnight under warm, humid conditions in a sealed plastic bag will show a mass of spore development. If samples taken for lab analysis are packed well (to keep the sward clean of any rootzone contamination), confirmation of this disease can be made within minutes of the lab receiving the sample because the fungus will have developed during shipment. The fungal spores will be moved (by wind, rain, machines, humans, birds) throughout the turf and each spore has the potential to start a new cycle of infection.
What weather conditions encourage disease development?
With regard to disease development, the effects of temperature, leaf wetness duration and relative humidity are known to be highly interdependent (Uddin et al, 2003b). As the ambient temperature increases, the duration of leaf wetness required for fungal spore germination and infection decreases. Research has shown that at temperatures between 27 – 32°C, only 9h of leaf wetness is necessary to facilitate infection but at lower temperatures (20 – 23°C), over 21h is required (Kerns, 2018). At temperatures below 9°C, fungal growth tends to cease. Actual leaf wetness enables spore germination and infection of the leaf, as well as rapid expansion of developing foliar lesions, however high relative humidity will encourage mass fungal spore production on the leaf surface (Uddin et al, 2003b).
Initial infections will develop early in the spring months when temperatures are relatively cool, but these initial infection levels are low and will generally go unnoticed. With each cycle of infection, the fungal presence in the turf will increase (through increasing amounts of spore inoculum) and eventually, during the summer months as temperatures & humidity increase, the quantity of fungal inoculum will be so overwhelming that infection will result in visible symptoms of disease. Increasing temperatures shorten the time taken for the fungus to complete its life cycle and the number of infection cycles builds rapidly through the late summer. At that time of the year, susceptible swards are at an elevated risk of disease development and most likely, if left un-treated the disease will completely kill affected turf.
The fungus is thought to survive saprophytically in decaying organic matter at the base of the turf and this could be a source of inoculum for future disease outbreaks. It is also possible that wind-borne annual infections occur but further research is needed to determine the relative contribution of each potential inoculum source (Uddin et al, 2003). However, with the rapid progress that is being made in molecular research, it should be possible to map the active fungal population in a specific turf area in consecutive years and compare the results to determine a more likely source of the outbreak.
How can we manage this disease?
Cultural conditions that will help to reduce disease development should focus on reducing relative humidity around the turf. The use of cooling fans in stadia, for example, will not only decrease air temperature but will also help to dry the leaf surface. Ensuring that the rootzone does not remain wet around the base of the turf will also help to decrease the humidity in the sward. It is important, especially in pure sand or high sand content rootzones, that the plant does not suffer drought conditions. Irrigation timing and application volume are critical factors during periods of potential disease activity, to ensure that the plant has access to sufficient water but that the leaf surface is kept dry for as long as possible. Ideally, susceptible turf should not be irrigated after 6pm so that the leaf surface remains dry overnight. There may be a case for using wetting agents to ensure water is available deeper in the rootzone and is held in the high sand content profiles.
Nutrient availability is also a factor that we can manage. There are reports that disease severity increases with increasing nitrogen availability and especially so if application is through a water-soluble source of nitrogen (Madeiras, 2020). In general, balanced nutrient availability at rates needed to maintain strong turf growth should reduce the plant’s susceptibility to disease and enable the plant to grow through minor disease outbreaks. Care should be taken not to encourage rapid, weak leaf development as this will be more susceptible to infection by the fungus. Observations have shown that GLS disease severity typically increases with increasing and rapidly available amounts of nitrogen (N) whereas controlled release fertilisers can help to reduce the risk of disease. Low rates of nitrogen applied at shorter intervals are less likely to encourage disease and ideally application rates should be kept below 1.25 g N / m² / application (Braitmaier, personal communication).
The turf should be maintained at an optimal height for the grass type but where disease symptoms begin to develop, the height of cut can be lowered slightly and the clippings removed. This reduction in the height of cut is the opposite to what would be recommended for minimising other Leaf Spot diseases (typically caused by Drechslera spp. or Bipolaris sp. fungi) but for all foliar outbreaks, it is best to remove clippings and thereby, remove potential inoculum. It is worth mentioning that if GLS is severely affecting the turf, clipping removal is unlikely to make a significant difference to the disease progression (such is the quantity of fungal spore inoculum during high disease pressure) (Bonos et al, 2006).
We know that young plants are susceptible to infection and therefore where disease has previously been confirmed on a site, be prepared for a likely infection at 4-5 weeks post emergence.
There has been much interest worldwide by turfgrass breeders to produce grass varieties (cultivars) that show reduced susceptibility to GLS disease and several such varieties of L. perenne are now available. Since 2000, Lolium varieties have been developed and tested in the USA for tolerance to GLS at the various sites of NTEP (National Turfgrass Evaluation Program: www.ntep.org) and at Rutgers University in New Brunswick (NJ) (https://turf.rutgers.edu/research/reports/). Breeders in the U.S. speak of so-called "GLS Resistant" varieties, but these varieties are not 100% resistant to GLS, they simply have reduced susceptibility to the disease.
Where this disease has previously developed or where there is concern that it could potentially develop, the use of these GLS tolerant varieties is highly recommended. It should be noted that varieties showing reduced susceptibility to GLS, have a darker green leaf colour than many L. perenne varieties and may not blend too well in an existing turf. That said, in Germany (since 2017) and in Austria (since 2018) many previously affected stadia have used the dark GLS tolerant varieties after their Koro-Renovation. High seed purity is essential for these tolerant varieties because ingress of the pale-green Poa annua or P. trivialis will be even more obvious through the turf.
One other option in disease-prone turf is the use of grasses that are not affected by this disease. For a sports field, the use of Poa pratensis might be considered but there are differential germination and establishment rates between P. pratensis and L. perenne that could make it an unsuitable choice in certain cases. Some groundsmen have achieved success in limiting GLS infection by seeding initially with 100% Poa pratensis (dark varieties) and some weeks later, overseeding with 100% GLS tolerant L. perenne. Other groundsmen are using a hybrid system consisting of a 30% Poa pratensis and 70% of GLS tolerant L. perenne mix of varieties for the summer renovation (Braitmaier, personal experience).
Fungicide applications will be effective against the causal fungus but as the disease becomes increasingly aggressive through the summer months, the relative efficacy of the fungicides is likely to be reduced. Early or preventative applications will be required to effectively manage summer outbreaks of the disease but there have already been reports of resistance or reduced susceptibility having been detected to the strobilurin and DMI fungicides, respectively (Bonos et al, 2006). Products with a multisite mode of action are likely to offer a more dependable, long term control and where available, such actives should be included in any fungicide programme.
There has been research into potential biological control of GLS by utilising bacteria populations or Trichoderma sp. formulations to naturally antagonise the pathogen population (Dammie, N., 2017). It is fair to say that against such an aggressive and potentially damaging turf disease, the limited and inconsistent evidence of any significant control would mean that at the moment, this option should not yet be relied upon to produce an effective management option. In the future, refinement of the products or formulations could result in products with improved efficacy and scientific support/backing.
When environmental conditions threaten severe disease potential, there is merit in trying to cool the turf or reduce the humidity around the turf canopy, especially in stadia situations, by using electric fans on the pitch. Cooling the turf is important because sustained high temperatures above 28°C promote rapid disease development. Integrated evaporative cooling fans can be used to reduce stadium air temperature to approximately 6-10°C below the ambient temperature. With the flexibility of being able to turn off the cooling system, the fan alone will help with general air movement and drying of the leaf.
Over recent years, there has been much use of UVC as an option to manage fungal spore inoculum (primarily spores of Microdochium nivale) on turf leaf surfaces and it could be of potential benefit in an integrated programme against Pyricularia spores too. Although it is unlikely that UVC would target inoculum around the base of the turf or fungal mycelium in plant debris, any positive reduction in spore activity on the leaf surface will reduce infection rates and thereby limit disease severity.
In stadia or on areas of L. perenne turf where this disease has not yet become established, it is worth investing in all options that aim to minimise the potential introduction of fungal spores or infected plant material that will act as a primary inoculum for the disease. Cleaning of all equipment that is used on different sites / pitches, either during renovations or for general turf maintenance, should be thoroughly cleaned and disinfected with special disinfection systems before being allowed on to a new turf area (Photo.6).

Photo.6 Disinfection system with foam (photo: grashobber GmbH & Co. KG, Germany)
Even the smallest amount of infected plant material could start a disease epidemic that may result in complete loss of the turf. Similarly, it is prudent to ensure that all footwear is cleaned before entry is allowed on to the turf (Photo.7).

Photo.7 Disinfection mat (photo: grashobber GmbH & Co. KG, Germany)
Spores of this fungus can easily be moved between sites in debris from affected areas. Disinfectant mats may be a worthwhile consideration for use on especially sensitive sites or where foot traffic is difficult to control. With air-borne spore movement, these mats on their own will not guarantee disease prevention but they can be used as another piece in an integrated management programme.
With recent advances in molecular biology, there is the possibility in the future to get near ‘real-time’ analysis of microbial communities. New technology can detect extremely small quantities of DNA belonging to a particular genus or species and could be used to confirm the presence of pathogens on, or in, turf that appears strong and healthy (Bronzato et al., 2018; Villari et al, 2017, Kumar et al, 2021). It is also possible to analyse spores collected in sticky traps using the same technologies & assess the risk to turf from air-borne sources of infection. Molecular biology has the distinct advantage of species level specificity & excellent sensitivity. Therefore, it is well suited to surveillance programmes and preventative strategies. Although new approaches to pathogen surveillance and validation of new technology will take time, an early warning system could be as simple as wiping the turf and assessing for any DNA of the fungus on the wiped swab.
In conclusion
GLS is a fungal disease that poses a potentially serious threat to managed L. perenne turf. If left unchecked and under ideal weather conditions for fungal development, the disease could kill a stadium pitch within a couple of days. It is critical that turf is monitored for the early signs of infection and that suspected disease outbreaks are confirmed by analysis. Due to the risk of confusion with various other diseases, e.g. Pythium and with drought stress, rapid and accurate diagnosis is required when the first symptoms appear because every minute counts with this aggressive disease. Once present in any turf area, the fungus will complete successive cycles of infection, building the level of spore inoculum with each cycle until eventually, the turf simply dies. By managing turf strength (through appropriate nutrient input), turf quality (by using cultivars with reduced susceptibility, monitoring height of cut, minimising the duration of leaf wetness and where possible, reducing air temperature / relative humidity) and where possible, implementing an effective fungicide programme (early / preventative application followed by a programmed approach that includes different fungicide modes of action), the chance of severe disease can be minimised. An integrated approach to disease management is advocated for all fungal disease problems but it is especially important when faced with a fungal disease that is as potentially damaging as GLS.
References:
Entwistle, K. (2020) Gray Leaf Spot on Perennial Ryegrass. Grounds Management magazine, October, pp22-23.
Wei, T. 2015. Epidemiology, phytopathological and molecular differentiation and leaf infection process of diverse strains of Magnaporthe spp. on wheat and rice. PhD Dissertation, Georg-August-University Göttingen, Germany. 169pp.
Tharreau, D., Fournier, E., Gladieux, P. & Lebrun, M-H. 2019. Pyricularia oryzae: quelques precisions taxonomiques. Phytoma, no. 723, p44.
Uddin, W., Viji, G. and Vincelli, P. 2003. Gray Leaf spot (Blast) of Perennial Ryegrass turf: an emerging problem for the turfgrass industry. Plant Disease, 87(8):880-889.
Butler, L. & Kerns, J. 2019. Gray Leaf spot in Turf. TurfFiles, NC State Extension Publication.
Uddin, W., Serlemitsos, K & Viji, G. 2003b. A temperature and leaf wetness duration-based model for prediction of gray leaf spot of perennial ryegrass turf. Phytopathology 93:336-343.
Kerns, J. 2018. Gray Leaf Spot on turfgrass. SportsField Management (official publication of the STMA), Jan 9, 2018.
Madeiras, A. 2020. Gray Leaf Spot on Ryegrass and Tall Fescue. Uni. Massachusetts Fact Sheet (on-line).
Bonos, SA., Murphy, JA. & Clarke, BB. 2006. Integrated control of Gray Leaf Spot on Perennial Ryegrass. Rutgers Cooperative Extension Fact Sheet FS1048.
Dammie, N. 2017. Biological Control of Gray Leaf Spot (Pyricularia grisea (Cooke) Sacc.) of Ryegrass. MSc in Plant Pathology. University of KwaZulu-Natal, Pietermaritzburg, 100pp.
Bronzato Badial, A., Sherman, D., Stone, A., Gopakumar, A., Wilson, V., Schneider, W. and King, J., 2018. Nanopore sequencing as a surveillance tool for plant pathogens in plant and insect tissues. Plant disease, 102(8), pp.1648-1652.
Villari, C., Mahaffee, WF., Mitchell, TK., Pedley, KF., Pieck, ML. and Hand, F 2017. Early Detection of Airborne Inoculum of Magnaporthe oryzae in Turfgrass Fields Using a Quantitative LAMP Assay. Plant Disease 101(1):170-177.
Kumar, S., Kashyap, PL., Mahapatra, S., Jasrotia, P & Singh, GP. 2021. New and emerging technologies for detecting Magnaporthe oryzae causing blast disease in crop plants. Crop Protection. Vol 143.
Further information
The writers can be contacted via the following email addresses:
Sabine Braitmaier: sabine.braitmaier@prosementis.de
Dr Deborah Cox: deborah@laganvalleyscientific.com
Dr Kate Entwistle: kate@theturfdiseasecentre.co.uk
