Introduction
Green plants require a number of essential chemical elements for normal growth and reproduction.
An essential element can be defined as
- one whose absence prevents a plant from completing its lifecycle; or
- one that has a clear physiological role.
These essential chemical elements are divided into two groups:
- Macronutrients - those elements that are required in relatively large amounts;
- Micronutrients - (also known as trace elements) those elements that are required in relatively small amounts.
There are 16 - 19 essential chemical elements, depending upon the method of classification of researchers.
Macronutrients
The first three macronutrients of Carbon, Hydrogen and Oxygen typically compose over 90% of the mass of a green plant and are not usually considered in any detail due to their abundance. The remaining number of macronutrients range from 6 - 9 depending upon classifications.
| Element | Chemical Symbol | Main available form taken up by the plant |
| 1. Carbon | C | These are obtained from air and water as CO2 and H20 (These are not considered mineral nutrients) |
| 2. Hydrogen | H | |
| 3. Oxygen | O | |
| 4. Nitrogen | N | NO3- ; NH4+ |
| 5. Phosphorus | P | H2PO4- ; HPO42- |
| 6. Potassium | K | K+ |
| 7. Calcium | Ca | Ca2+ |
| 8. Magnesium | Mg | Mg2+ |
| 9. Sulphur | S | SO42- |
| Chlorine and Iron are sometimes classed as either a macronutrient or micronutrient | ||
| 10. Chlorine | Cl | Cl- |
| 11. Iron | Fe | Fe2+ ; Fe3+ |
| Modern studies may also class Silicon as a macronutrient. | ||
Micronutrients
Typically 6 - 9 elements are classed as micronutrients.
| Element | Chemical Symbol | Main available form taken up by the plant |
| 10. Chlorine | Cl | Cl- |
| 11. Iron | Fe | Fe2+ ; Fe3+ |
| 12. Boron | B | H2BO3- |
| 13. Copper | Cu | Cu2+ |
| 14. Manganese | Mn | Mn2+ |
| 15. Molybdenum | Mo | MoO4- |
| 16. Zinc | Zn | Zn2+ |
| Sodium may be classed as a micronutrient for C4 plants | Na | Na+ |
| Nickel is sometimes classed as a micronutrient in modern studies, although requirements are minuscule. | ||
Classification due to Biochemical Function
Some influential researchers, however, have suggested that the essential elements should be classified into 4 groups which reflect the biochemical role and physiological function of the nutrients within the plant, as follows:
- Nutrients that are part of carbon compounds: [N, S];
- Nutrients that are important in energy storage or structural integrity: [P, Si, B];
- Nutrients that remain in ionic form: [K, Ca, Mg, Cl, Mn, Na];
- Nutrients that are involved in redox reactions (i.e. energy supplying processes): [Fe, Zn, Cu, Ni, Mo].
Mineral Nutrition
Mineral nutrition is the term used to describe the study of how plants obtain and use these essential elements as the majority of them arise from soil minerals.
Soil weathering, supplemented by deposits from rainfall and irrigation water, provides the majority of nutrients required by turfgrasses.
Some main nutrients are not generally available in sufficient quantities for turfgrass situations and these have to be provided as a fertiliser or within top-dressings.
These are primarily Nitrogen (N), Phosphorus (P) and Potassium (K).
Other elements that may be applied to turf include Iron (Fe) as a turf tonic, especially for fine turf situations, and Magnesium (Mg) which is occasionally applied to high sand content rootzones.
The effects of these five elements on turfgrass are provided in another article on turfgrass nutrients.
Nutrients within Sand
Even sand which is a main component of most top-dressings and is often considered inert can contain a surprising amount of nutrients.
| It is interesting to note that the chemical analysis of some sands has been found to contain the following elements (all figures are in parts per million, ppm): | |
| Phophorus: | 0 - 14.4 |
| Iron: | 1.0 - 138 |
| Magnesium: | 1.1 - 168 |
| Potassium: | 1.5 - 230 |
| Calcium: | 23.2 - 100+ |
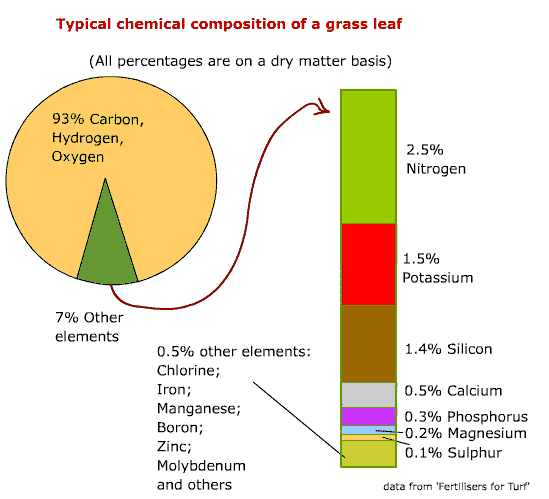
Chemical Composition of a Grass Leaf
References and Further Reading
- Simon, E.W. et al, (1973), 'Lowson's Botany', Unwin Hyman
- Russell, E.W. (1973), 'Soil Conditions and Plant Growth', Longman
- Lawson, D.M. & Baker, S.W., (1987), 'The nutrient content of sands used in turf culture in the United Kingdom', Journal of the Sports Turf Research Institute, Vol.63, 49-56
- Baker, S.W., (1990), 'Sands for Sports Turf Construction and Maintenance', STRI
- Lawson, D.M., (1991), 'Fertilisers for Turf', STRI
- Adams, W.A. & Gibbs, R.J. (1994), 'Natural Turf for Sport and Amenity: Science and Practice', CAB International
- Bailey, J (Ed), (1999), 'The Penguin Dictionary of Plant Sciences', Penguin Books
- Taiz, L. & Zeiger, E. (2002),'Plant Physiology', 3rd Edn, Sinauer Associates, Inc.
